Learn WHO guidelines for analytical method transfer with SU and RU duties in technology transfer process in industrial pharmacy.
Analytical Method Transfer in Technology Transfer (TT)
- Analytical methods transfer should cover all the analytical tests related to product to be transferred with the registered description.
- Analytical methods used for testing pharmaceutical products, packaging components, cleaning (residue) samples and starting materials.
- It should be applied at the testing laboratory, prior to the Receiving Unit (RU) conducting process validation studies. Process validation samples should be tested at the Sending Unit (SU), Receiving Unit (RU) or a third laboratory.
Analytical Method Transfer Protocols
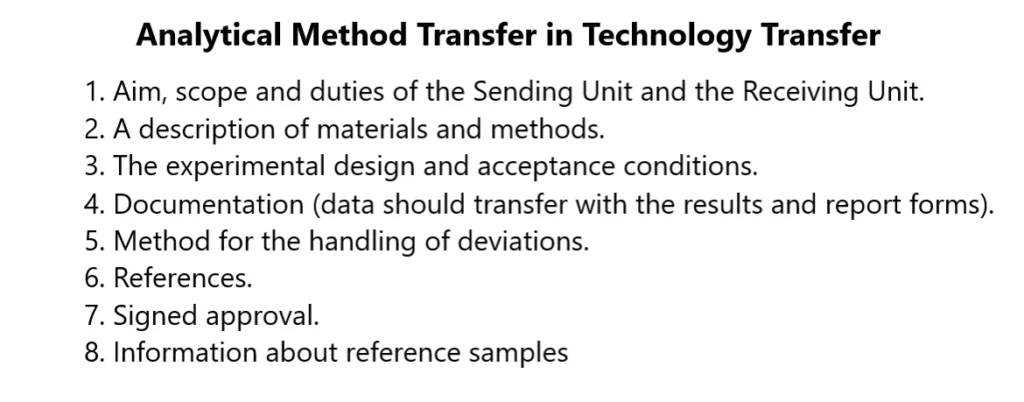
This analytical method transfer protocol should contain details of the:
- Aim, scope and duties of the Sending Unit and the Receiving Unit.
- A description of materials and methods.
- The experimental design and acceptance conditions.
- Documentation (data should transfer with the results and report forms).
- Method for the handling of deviations.
- References.
- Signed approval.
- Information about reference samples (initial materials, intermediates and finished products).
Duties of Sending Unit (SU) for the transfer of analytical methods
- Provide procedure-specific training for analysts and other quality control personnel.
- Help in analyzing Quality Control testing results.
- Outline procedures for testing specific products, starting materials and cleaning samples.
- Outline the experimental design, sampling procedures and acceptance conditions.
- Deliver any validation reports for procedures under transfer and validate their robustness.
- Offer specifications of the equipment used and any standard reference samples.
- Offer approved methods used in testing.
- Assess and approve transfer reports.
Duties of Receiving Unit
- Review the analytical methods offered by the SU and accept the acceptance conditions before performing the transfer protocol.
- Make sure that the essential equipment for QC is present and qualified at the RU site.
- Ensure trained and experienced personnel perform the analytical tests.
- Offer a documentation system to record receipt and testing of samples as per the needed specifications.
- Perform the transfer protocol.
- Achieve the suitable level of validation to assist the execution of procedures.
- Make and attain approval of transfer reports.
Ensure sufficient training and record all procedures and outcomes.
Expect referencing compendial monographs (e.g., British Pharmacopoeia, International Pharmacopoeia, European Pharmacopoeia and United States Pharmacopoeia).
Also read Difference between qualification and validation. General consideration for pilot plant scale up technique.
