Learn how to perform assay of aspirin. It also known as acetylsalicylic acid used to relieve pain, reduce inflammation and reduce fever.
How to perform assay of aspirin?
It also known as acetylsalicylic acid. This medication used treat relieve pain, inflammation and fever.
It belongs to the class of drugs known as nonsteroidal anti-inflammatory drugs (NSAIDs).
Physiochemical properties
| Chemical Formula | C9H8O4 |
| Molecular Weight | 180.16 g/mol |
| Melting Point | 136°C |
| Boiling Point | Decomposes before boiling. |
| Solubility | Slightly soluble in water, ethanol, chloroform. |
| pKa (acid dissociation constant) | 3.5 (carboxylic acid group) |
| Appearance | White crystalline powder |
| Odor | Odorless |
| Density | ~1.4 g/cm³ (at 20°C) |
| LogP (Partition Coefficient) | ~1.4 (estimate) |
| pH (1% Solution) | ~3.0 – 4.5 |
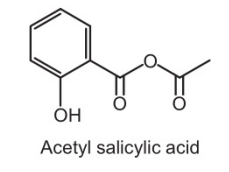
Aspirin structure
Requirements
- Analytical balance for accurate weighing.
- Beakers, flask and pipettes for preparing and mixing solutions.
- Heating source for boiling the mixture.
- Cooling equipment.
- Gloves, goggles and lab coat.
- Aspirin sample (approximately 1.5 grams).
- Ethanol (95%) for dissolving the aspirin.
- 0.5 M sodium hydroxide for titration.
Principle
The assay of aspirin by titration is based on acid-base titration principles. In this assay, aspirin (acetylsalicylic acid) act as the weak acid and it is titrated with a strong base (sodium hydroxide, NaOH) of known concentration.
The primary principle involves the neutralization reaction between the acidic aspirin and the basic sodium hydroxide.
This is freely soluble in ethanol (95%), chloroform and in ether also slightly soluble in water.
Aspirin assay procedure
- Weigh accurately about 1.5 g of aspirin sample.
- Then dissolved it in 15 ml of ethanol (95%).
- And add 50.0 ml of 0.5 M sodium hydroxide.
- Now, boil gently for 10 min and cool.
- Titrate the excess of alkali with Phenol red indicator.
- Perform a blank determination to see the difference between the titration represent the volume of sodium hydroxide consumed.
Each ml of 0.05M NaOH = 0.04504g of C9H8O.
Uses: It is used in the treatment of headache.
ALSO READ Assay of Ibuprofen. Assay of atropine sulphate. Assay of phenobarbitone.
