Learn What is flocculated and deflocculated suspension? Explore the difference between them with definitions and examples.
What is Flocculated suspension?
A flocculated suspension refers to a mixture in which particles are loosely bound together to form larger aggregates or clusters, resulting in the formation of visible flocs or clumps. These flocs are held together by weak physical interactions, such as van der Waals forces or electrostatic attractions. Flocculation can occur due to the presence of flocculating agents or conditions that promote particle aggregation.
Examples of flocculated suspensions include:
- Clay in water: When clay particles are suspended in water, they can flocculate, forming visible clumps or flocs.
- Sewage treatment: During the treatment of wastewater, flocculation is often employed to help aggregate impurities and facilitate their removal.
- Ceramic slip: In the production of ceramics, a flocculated suspension of clay and other additives is used to achieve the desired consistency for casting or molding.
What is deflocculated suspension?
A deflocculated suspension is a mixture where particles are dispersed as individual entities throughout a liquid or gas, without significant clustering or aggregation. The particles remain separate and do not form visible flocs or clumps.
For example of deflocculated suspension can be seen in a well-dispersed ink solution, where the pigment particles are uniformly distributed and do not settle or form visible aggregates over time.
Differences between flocculated and deflocculated suspension
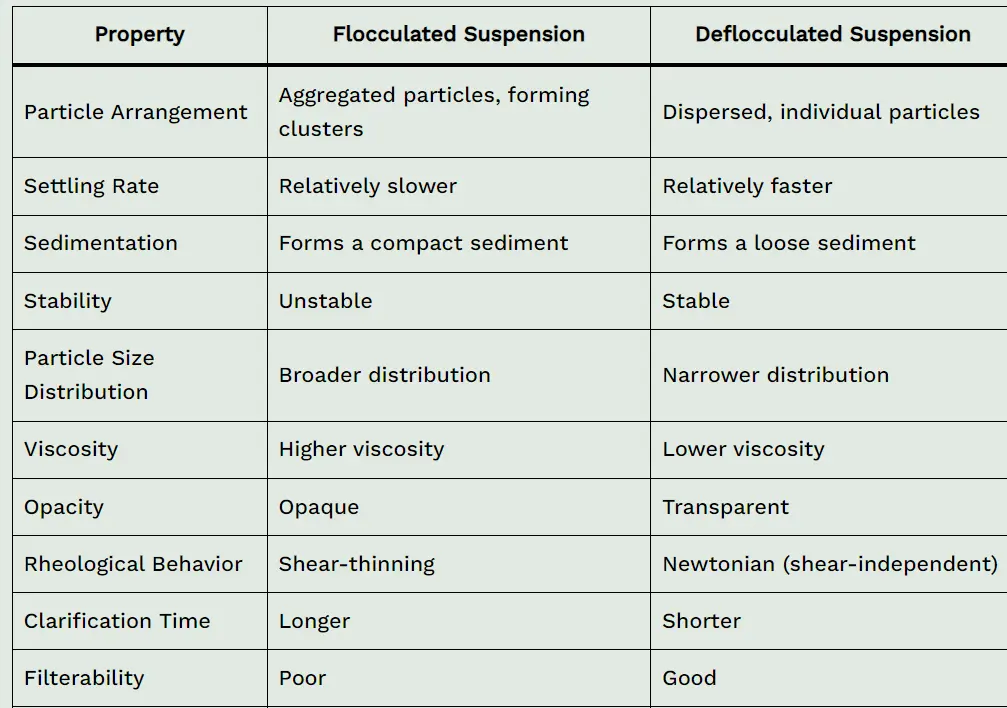
| Property | Flocculated Suspension | Deflocculated Suspension |
|---|---|---|
| Particle Arrangement | Aggregated particles, forming clusters | Dispersed, individual particles |
| Settling Rate | Relatively slower | Relatively faster |
| Sedimentation | Forms a compact sediment | Forms a loose sediment |
| Stability | Unstable | Stable |
| Particle Size Distribution | Broader distribution | Narrower distribution |
| Viscosity | Higher viscosity | Lower viscosity |
| Opacity | Opaque | Transparent |
| Rheological Behavior | Shear-thinning | Newtonian (shear-independent) |
| Clarification Time | Longer | Shorter |
| Filterability | Poor | Good |
| Homogeneity | Non-uniform distribution of particles | Uniform distribution of particles |
| Particle Interactions | Strong particle-particle interactions | Weak particle-particle interactions |
| Re-dispersion Ability | Difficult to re-disperse | Easy to re-disperse |
| Clarity | Less clear or turbid | Clear or less turbid |
| Settling Volume | Higher settling volume | Lower settling volume |
| Fluidity | Higher resistance to flow | Better flowability |
| Shelf Life | Shorter shelf life due to sedimentation | Longer shelf life due to stability |
| Surface Area | Lower specific surface area | Higher specific surface area |
| Particle Size Separation | Difficult to separate particles by size | Easier separation of particles by size |
| Application Suitability | Suitable for certain processes or industries | Suitable for certain processes or industries |
| Aggregate Size | Larger aggregate size | Smaller aggregate size |
| Dispersion Stability | Unstable, prone to settling and re-aggregation | Stable, resists settling and re-aggregation |
| Shear Resistance | Relatively lower shear resistance | Higher shear resistance |
| Rheology Control | Limited control over rheological properties | Better control over rheological properties |
| Sedimentation Rate | More prone to sedimentation over time | Reduced sedimentation over time |
| Particle Packing Efficiency | Lower packing efficiency | Higher packing efficiency |
Also read What are suspensions and examples? Difference between monophasic liquids vs biphasic liquids.
