Here is a simplified table highlighting the differences between Qualification and Validation in industrial pharmacy.
Difference between qualification and validation
Qualification defines as the process of checking and documenting whether equipment, systems or processes meet specific standards and requirements for their intended use. It ensures that everything is up to the necessary standards and functions correctly.
Validation defines as the process of gathering proof, in the form of documented evidence, that a manufacturing process consistently creates products that meet the predetermined quality standards. It ensures that the process consistently produces high-quality products as intended.
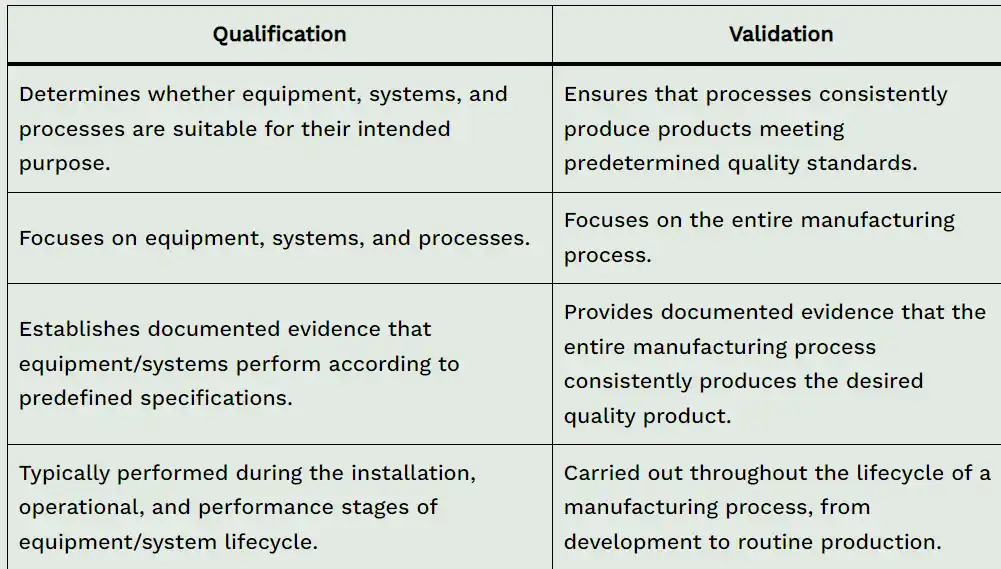
| Qualification | Validation |
|---|---|
| Determines whether equipment, systems, and processes are suitable for their intended purpose. | Ensures that processes consistently produce products meeting predetermined quality standards. |
| Focuses on equipment, systems and processes. | Focuses on the entire manufacturing process. |
| Establishes documented evidence that equipment/systems perform according to predefined specifications. | Provides documented evidence that the entire manufacturing process consistently produces the desired quality product. |
| Typically performed during the installation, operational, and performance stages of equipment/system lifecycle. | Carried out throughout the lifecycle of a manufacturing process, from development to routine production. |
| Involves testing and verifying individual components and subsystems of equipment/systems. | Involves testing and evaluating the entire manufacturing process, including inputs, controls, and outputs. |
| Includes qualification activities such as Installation Qualification (IQ), Operational Qualification (OQ), and Performance Qualification (PQ). | Includes validation activities such as Process Validation, Cleaning Validation, and Analytical Method Validation. |
| Focuses on ensuring equipment/systems meet predefined standards and specifications. | Focuses on ensuring the manufacturing process consistently produces products of the desired quality. |
| Results in documented evidence of equipment/system suitability and compliance. | Results in documented evidence of process performance and compliance. |
| Assesses the functionality and performance of equipment/systems. | Assesses the reliability and robustness of the entire manufacturing process. |
Please note that this table provides a simplified overview, and the actual implementation and specific requirements may vary based on regulatory guidelines and industry standards.
Also read Pilot plant scale up technique.
