Learn Granularity of Technology Transfer (TT) Process and the materials such as API, excipients, finished products and packaging procedure.
Granularity of Technology Transfer
The granularity of technology transfer refers to the level of detail or specificity at which technology is transferred from one entity to another. It refers to the extent to which the knowledge, information and expertise associated with a particular technology are shared and transferred.
For complete granularity of technology transfer materials such as API, excipients, finished products and packaging procedure are discussed below.
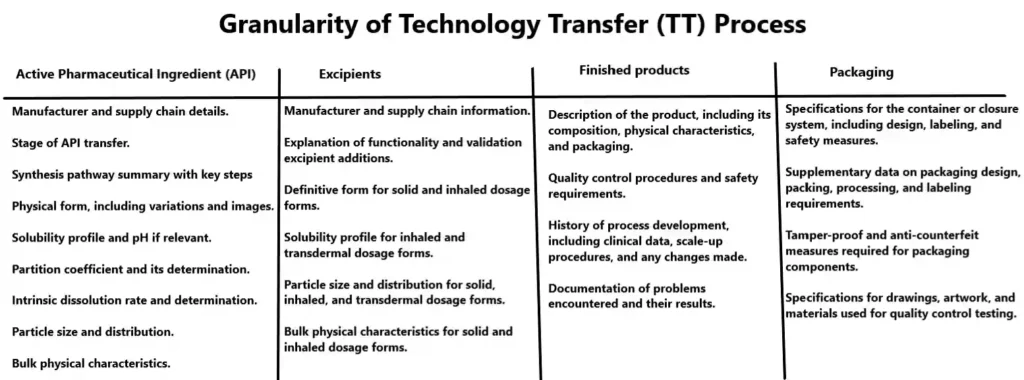
Active Pharmaceutical Ingredient (API)
When transferring the Active Pharmaceutical Ingredient (API), the supplying company (SU) should provide the following information in a simplified manner:
- Manufacturer and supply chain details.
- Stage of API transfer.
- Synthesis pathway summary with key steps and controls.
- Physical form, including variations and images.
- Solubility profile and pH if relevant.
- Partition coefficient and its determination.
- Intrinsic dissolution rate and determination.
- Particle size and distribution.
- Bulk physical characteristics.
- Water content and special handling requirements.
- Microbiological considerations.
- Compliance with standards.
- Specifications and validation for quality control.
In summary, the SU should share concise information about the API’s manufacturing, physical properties, solubility, particle size, and compliance with quality standards.
Excipients
When transferring excipient information, the supplying company (SU) should provide the following details in a simplified manner:
- Manufacturer and supply chain information.
- Explanation of functionality and validation for antioxidant, preservative, or other excipient additions.
- Definitive form for solid and inhaled dosage forms.
- Solubility profile for inhaled and transdermal dosage forms.
- Particle size and distribution for solid, inhaled, and transdermal dosage forms.
- Bulk physical characteristics for solid and inhaled dosage forms.
- Melting point range for semi-solid or topical dosage forms.
- pH range for different dosage forms.
- Water content, hygroscopicity, and special handling requirements for solid and inhaled dosage forms.
In summary, the SU should provide concise information about excipient details such as manufacturer, functionality, physical properties, and specific considerations for different dosage forms, including solubility, particle size, pH range, and water content.
Finished products
When transferring finished product information, the supplying company (SU) should provide the following simplified details:
- Description of the product, including its composition, physical characteristics, and packaging.
- Quality control procedures and safety requirements.
- History of process development, including clinical data, scale-up procedures, and any changes made.
- Documentation of problems encountered and their results.
In summary, the SU should provide concise information about the finished product, its composition, manufacturing process, quality control measures, and any relevant development history.
Packaging
- Specifications for the container or closure system, including design, packing, processing, and labeling requirements.
- Tamper-proof and anti-counterfeit measures required for packaging components.
- Specifications for drawings, artwork, and materials used for quality control testing of packaging components.
- The receiving company (RU) should conduct a suitability study to ensure the packaging components are appropriate.
- Sufficient protection: Packaging should prevent drug degradation due to environmental factors.
- Safety: Packaging should not release unwanted substances into the product.
- Compatibility: Packaging should not interact with the drug, potentially affecting its quality.
- Performance: Packaging should function properly for effective drug delivery.
In summary, the supplying company (SU) should provide specifications for the packaging materials, and the receiving company (RU) should conduct a suitability study to ensure the packaging components meet requirements for protection, safety, compatibility, and performance.
