Explore pilot plant scale up techniques for liquid orals products, including formulation optimization, process validation and more.
What is Pilot Plant Scale Up Techniques for Liquid Orals?
Pilot plant scale-up techniques for liquid orals refer to the methods and processes used to transition a laboratory-scale formulation of liquid oral products to large-scale commercial production. ]
These techniques involve optimizing the formulation, validating the manufacturing process and ensuring quality control measures are in place to maintain product stability, efficacy, and regulatory compliance during the scale-up process.
Key aspects of pilot plant scale up techniques for liquid orals
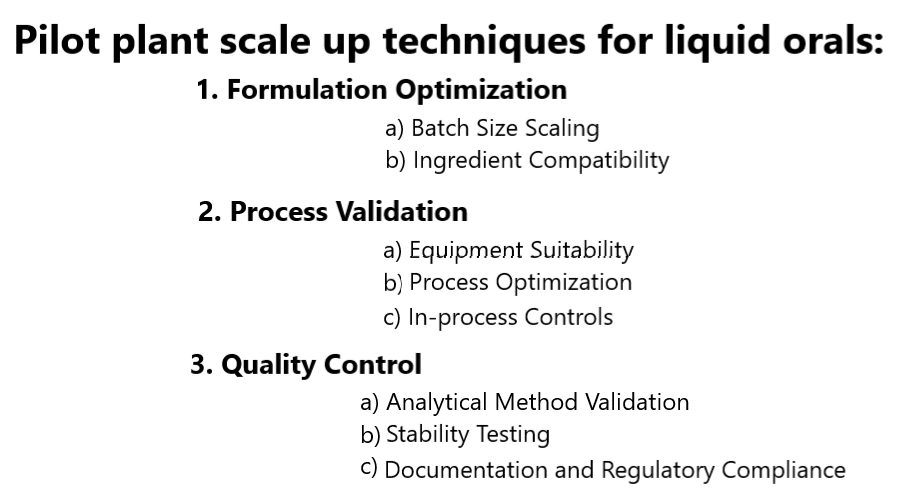
1. Formulation Optimization
- Batch Size Scaling: To begin the scale-up process, it is essential to determine the appropriate batch size for pilot plant production. This involves calculating the scaling factor based on the desired commercial batch size. Factors such as mixing efficiency, equipment limitations, and production time should be considered to ensure a smooth transition.
- Ingredient Compatibility: As the batch size increases, it becomes crucial to re-evaluate the compatibility of the ingredients used in the formulation. Interactions between different components can lead to phase separation, precipitation or changes in viscosity. Conducting compatibility studies and adjusting the formulation accordingly is vital to maintain product stability.
2. Process Validation
- Equipment Suitability: Pilot plant scale-up requires selecting equipment that closely resembles the commercial production setup. Ensure that the equipment used during the pilot plant stage matches the specifications of the larger-scale equipment to minimize variations in process parameters.
- Process Optimization: While scaling up, it is important to optimize the manufacturing process to achieve consistent product quality. This involves evaluating critical process parameters such as mixing speed, temperature, and duration. Statistical tools, such as Design of Experiments (DOE), can be employed to identify the ideal operating conditions.
- In-process Controls: Implementing in-process controls throughout the pilot plant scale-up process is crucial for ensuring the quality and uniformity of the liquid oral product. Regular sampling and testing at various stages enable real-time adjustments and allow for early detection of any deviations from the desired product attributes.
3. Quality Control
- Analytical Method Validation: Validating the analytical methods used to assess the quality of the liquid oral product is essential during scale-up. Analytical techniques such as high-performance liquid chromatography (HPLC) and spectrophotometry should be validated to ensure accurate and reliable results.
- Stability Testing: Conducting stability studies under accelerated and long-term conditions is critical to determine the product’s shelf life and ensure its integrity over time. These studies help identify any physical or chemical changes that may occur during storage and provide insights for product formulation adjustments, if necessary.
- Documentation and Regulatory Compliance: Thorough documentation of the entire pilot plant scale-up process is essential for regulatory compliance and future reference. This includes recording all experimental parameters, manufacturing steps, and analytical results. Adhering to good manufacturing practices (GMP) and regulatory guidelines is crucial throughout the scale-up process.
Conclusion
Pilot plant scale up techniques for liquid orals are vital for ensuring the successful transition from laboratory-scale formulation to commercial production. Formulation optimization, process validation, and quality control are key pillars of this scale-up process. By meticulously evaluating and adjusting critical parameters, manufacturers can minimize risks, maintain product quality, and meet regulatory requirements. Implementing these techniques effectively ensures the efficient and reliable production of liquid oral products on a larger scale, benefiting both the manufacturer and the end consumer.
Also read Pilot Plant Scale Up Techniques for Solid Dosage Forms.
