Learn how to prepare triphenyl imidazole from benzyl and benzaldehyde. It has antibacterial and anti-inflammatory activities.
How to prepare triphenyl imidazole?
It is a derivative of imidazole. Imidazole and its derivatives have various applications in organic chemistry, pharmaceuticals, and biochemistry.
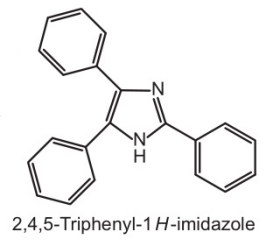
Triphenyl imidazole structure
Requirements
- Benzyl (1 gram).
- Ammonium acetate (1 gram).
- Benzaldehyde (2 milliliters).
- Glacial acetic acid (2 milliliters).
- Round-bottom flask.
- Reflux apparatus.
- Water (150 milliliters).
- Ammonium hydroxide or sodium carbonate.
- Toluene.
- Methanol.
Principle
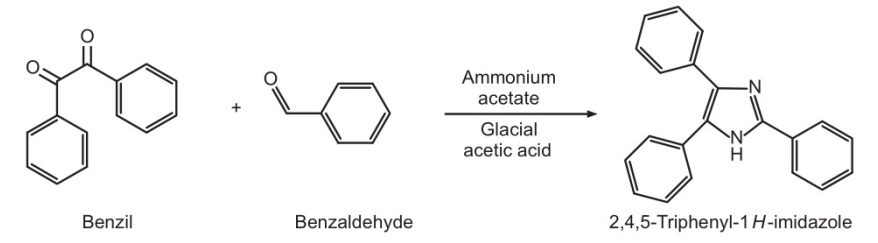
2,4,5-Triphenyl-1H-imidazole was prepared from benzyl, ammonium acetate and benzaldehyde in the presence of glacial acetic acid.
Procedure
- Take benzyl (1 gm), ammonium acetate (1 gm), benzaldehyde (2 ml), glacial acetic acid (2 ml) in round bottom flask and reflux for 3 hours.
- Allow reaction mixture to stand to room temperature to attain room temperature.
- Add 150 ml of water and filter.
- Neutralize the filtrate with either ammonium hydroxide or sodium carbonate. This will result in a solid, pasty mass. Filter it again.
- Then the solid mass is washed with toluene and recrystallize from methanol.
- The final melting point of 2,4,5-tripheny-1H-imidazole is 274-278°C.
Now calculate the percentage yield and theoretical yield and write the report:

Uses: It is mainly used as an antimicrobial agent and anti-inflammatory agent.
ALSO READ Preparation of chlorobutanol from acetone. Preparation of 7-Hydroxy 4-methyl coumarin.
