Learn about regulatory requirements for drug approval worldwide. Here is the step-by-step process to bring new medicines to market.
Regulatory Requirements for Drug Approval
When it comes for approval of a new medicine, there are several rules to follow known as regulatory requirements for drug approval. Each country has its own unique regulatory requirements. This means the approval process can be challenging for a new drug to market globally.
Steps of drug approval
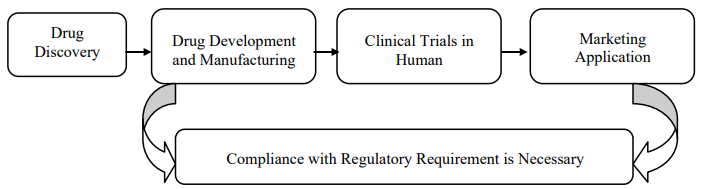
- Drug Discovery and Optimization: Scientists identify a drug molecule for treatment of specific disease. They work to improve and optimize it.
- Pre-Clinical Trials: Before testing on humans, the drug molecule undergoes safety and efficacy tests on animals. This step ensures it is not harmful and works effectively.
- Approval for Clinical Trials: To proceed to human trials, researchers must take approval from the government authority in each country where they plan to conduct these trials.
- Clinical Trials in Phases: Human trials are conducted in four phases to ensure its safety and effectiveness. Dosage for humans is adjusted during these phases.
- Marketing Authorization Application (MAA): Once clinical trials completed safety and efficacy test, a formal application for marketing authorization is submitted to the appropriate regulatory authority.
- Approval and Post-Marketing Studies: The regulatory authority reviews the application. They make sure the drug meets safety and efficacy criteria. If approved, the drug can be marketed. Post-marketing studies may also be required to monitor ongoing safety and effectiveness.
You may also like to read What are the roles of Regulatory Affairs Department?
FAQ
- What are regulatory requirements for drug approval?
Answer: These are a set of rules and processes that must be followed to gain approval for a new medicine.
- What is the purpose of pre-clinical trials in the drug approval process?
Answer: Pre-clinical trials are conducted on animals to ensure the safety and effectiveness of a drug molecule. These tests help determine if the drug is harmful and its effectiveness before human trials.
- Why clinical trials need approval from government authorities in each country?
Answer: Approval from government authorities in each country is required to conduct clinical trials on humans. Each country has its own regulatory processes and this step ensures compliance with local regulations.
- How many phases of clinical trials are there and what is their primary focus?
Answer: There are four phases of clinical trials. Their primary focus is to assess the safety and effectiveness of the drug in humans.
- What happens after a Marketing Authorization Application (MAA) is submitted?
Answer: After submitting an MAA, the regulatory authority reviews the application to ensure the drug meets safety and efficacy criteria. If approved, the drug can be marketed.
