Learn how to perform simultaneous estimation of ibuprofen and paracetamol by UV spectroscopy in laboratory.
Estimation of ibuprofen and paracetamol
Aim: To carry out simultaneous estimation of Ibuprofen and Paracetamol using UV spectroscopic method.
Requirements
Chemicals: Ibuprofen powder, paracetamol powder, ethanol, water, methanol Apparatus: UV spectrophotometer, measuring cylinder, volumetric flask, electronic balance, filter paper
Theory
- Ibuprofen chemically known as 2-(4-(2-methylpropyl) phenyl) propanoic acid. Ibuprofen is a nonsteroidal anti-inflammatory drug class that is used for treating pain, fever and inflammation. It acts by inhibiting COX-2 which decreases the synthesis of prostaglandins involved in mediating pain and inflammation.
- Paracetamol chemically known as N-(4-hydroxyphenyl) acetamide. It is an analgesic and antipyretic agent. It acts primarily in the CNS, increasing the pain threshold by inhibiting COX-1, COX-2 and COX-3 isoforms of enzymes.
- The combination of ibuprofen and paracetamol used for the treatment of pain, fever and inflammation associated with musculoskeletal and joint disorders.
Procedure
1) Selection of solvent and wavelength:
- Check the solubility of ibuprofen and paracetamol by using solvents like ethanol, water and methanol.
- Record the UV spectrum of these two drugs.
- In ethanol, these drugs have maximum absorbance as compared to other two.
- After recording absorbance, select 220 and 240 nm wavelengths, which is Amax for ibuprofen and paracetamol respectively.
2) Preparation of standard stock solution:
- Weigh Ibuprofen and paracetamol (10 mg each) separately
- Now transfer into 100 ml volumetric flask
- Then dissolved in ethanol to get concentration of 100μg/ml.
3) Analysis of formulation:
- Weigh 20 tablets, each should contain 40 mg of ibuprofen and paracetamol.
- Calculate the average weight of the tablets.
- Dissolve the weighed tablets in ethanol to achieve the desired concentrations.
- Adjust the volume to reach the desired concentrations.
- Record absorbances at 220 nm for ibuprofen and 240 nm for paracetamol.
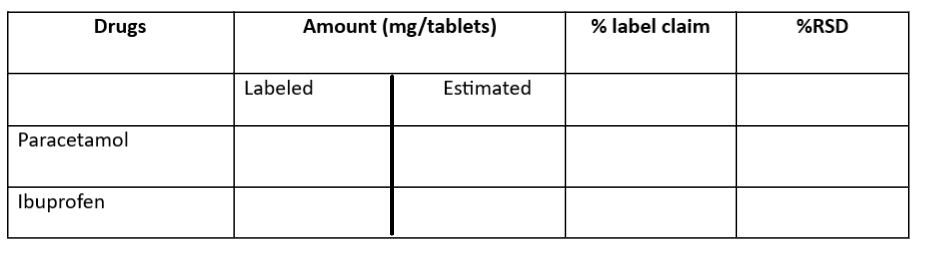
Calculation
The amount of ibuprofen a paracetamol was calculated using simultaneous equation method;
- Cx= A2ay1-A1ay2/ax2ay1 – ax1ay1
- Cy= A1ax2-A2ax1/ax2ay1-ax1ay1
X= Paracetamol, Y = Ibuprofen, Cx = Conc. of PCM, Cy= Conc. of ibuprofen, ax1 = Absorptivity of PCM at 240 nm, ax2 = Absorptivity of PCM at 220 nm, ay1 = Absorptivity of IBU at 220 nm, ay2= Absorptivity of IBU at 240 nm, A1 = Absorbance of sample at Lamda1, A2= Absorbance of sample at lambda2.
You may also like to read Determine absorption maxima of Paracetamol. Estimation of Sulfanilamide by Colorimetry.
SAQ
- Question: Which chemicals are required for estimation of ibuprofen and paracetamol by UV spectroscopy?
- Answer: Ibuprofen powder, Paracetamol powder, ethanol, water, and methanol.
- Question: What is the principle of Ibuprofen in the body?
- Answer: It acts by inhibiting COX-2, which reduces the synthesis of prostaglandins involved in mediating pain and inflammation.
- Question: How does Paracetamol utilize its analgesic and antipyretic effects?
- Answer: It increases the pain threshold in the central nervous system by inhibiting COX-1, COX-2 and COX-3 isoforms of enzymes.
- Question: What is the purpose of selecting a solvent and wavelength in this experiment?
- Answer: The solvent and wavelength selection are important to maximize the absorbance of Ibuprofen and Paracetamol. Ethanol is chosen as the solvent, and wavelengths of 220 nm and 240 nm are selected as they represent the maximum absorbance for Ibuprofen and Paracetamol, respectively.
- Question: How standard stock solution prepared for Ibuprofen and Paracetamol?
- Answer: To prepare the standard stock solution, weigh 10 mg of Ibuprofen and Paracetamol separately and dissolve in ethanol to achieve a concentration of 100μg/ml.
- Question: What is the procedure for analyzing the formulation tablets in this experiment?
- Answer: Weigh the tablets, each containing 40 mg of Ibuprofen and Paracetamol. Calculate the average tablet weight and dissolve in ethanol to reach the desired concentrations. Now record the absorbances at 220 nm for Ibuprofen and 240 nm for Paracetamol.
- Question: Why is simultaneous estimation of Ibuprofen and Paracetamol important in pharmaceutical analysis?
- Answer: Simultaneous estimation is crucial for quality control and formulation development in pharmaceuticals.
- Question: What is the therapeutic application of combination of Ibuprofen and Paracetamol?
- Answer: For the treatment of pain, fever and inflammation associated with musculoskeletal and joint disorders.
