Learn how to prepare and synthesis of benzocaine (ethyl p-amino benzoate) from p-nitrobenzoic acid with preparation procedure and theory.
Benzocaine is a local anesthetic commonly used to relieve pain and discomfort on the skin or in the mucous membranes of the mouth and throat. It belongs to a class of medications known as local anesthetics. It works by blocking nerve signals in the affected area, thereby reducing the sensation of pain.
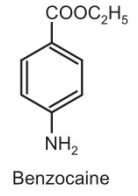
| Chemical Formula | C9H11NO2 |
| Molecular Weight | 165.19 g/mol |
| Melting Point | 88-90°C |
| Boiling Point | Decomposes |
| Density | 1.17 g/cm³ at 20°C |
| Solubility in Water | Slightly soluble |
| Solubility in Organic Solvents | Soluble in organic solvents like ethanol, acetone and chloroform |
| pKa (Acid Dissociation Constant) | Approximately 2.5 |
| Appearance | White, crystalline powder |
Preparation and synthesis of Benzocaine
For preparation these requirements are needed:
- p-Nitrobenzoic acid
- Absolute ethanol
- Conc. H2SO4
- Carbon tetrachloride
- Magnesium sulphate
- Ethyl p-nitrobenzoate
- Conc. HCl
- Ethyl acetate.
Principle
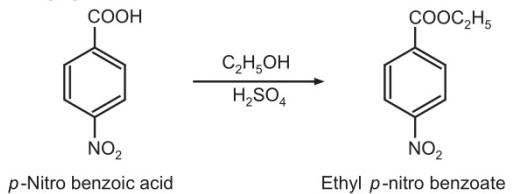
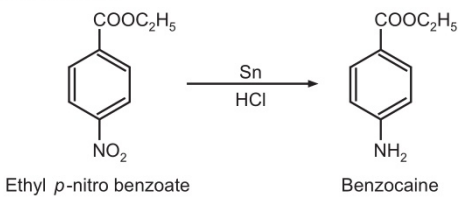
p-Nitrobenzoic acid is esterified to ethyl p-nitrobenzoate by heating with absolute ethanol and few drops of sulphuric acid. Then, ethyl p-nitro benzoate is reduced with granulated tin and hydrochloric acid to Benzocaine.
Synthesis of benzocaine
Step-I: Synthesis of ethyl p-nitrobenzoate:
Step-II:
Preparation procedure of benzocaine synthesis
Step-I:
- Weigh out 4.083 grams of p-nitrobenzoic acid.
- Combine it with 15 ml of absolute ethanol and 0.3 ml of concentrated H2SO4 in a round-bottomed flask.
- Reflux the mixture for 4 hours.
- After refluxing, distill off excess alcohol and let the mixture cool.
- Transfer the residue into a separating funnel containing 25 ml of water.
- Rinse the flask with a few ml of water, adding it to the separating funnel.
- Add about 5 ml of CCl4 and shake the mixture vigorously.
- Allow it to stand, and you’ll observe a heavy solution of methyl benzoate in CCl4 separating rapidly at the bottom of the funnel.
- Carefully run off the lower layer, discarding the upper layer.
- Return the methyl benzoate to the funnel and shake it with a strong solution of sodium hydrogen carbonate until no more CO2 evolves.
- Wash the mixture once with water.
- Dry it by transferring it to a small dry conical flask containing 2-3 gm of MgSO4.
- Stopper the flask, shake for 5 minutes, and let it stand for at least half an hour with occasional shaking.
- Filter the solution directly into a round-bottomed flask fitted with a still head, a 360 thermometer and an air condenser.
- Add a few boiling chips and distill from an air bath, gradually increasing the temperature until you collect the crude ethyl p-nitrobenzoate.
Step-II:
- In a 500 ml round-bottom flask with a reflux condenser, place 4.85 gm of ethyl p-nitrobenzoate and 4.5 gm of granulated tin.
- Measure 10 ml of concentrated HCl.
- Pour about 1.5 ml of HCl into the condenser.
- Shake the flask steadily; the mixture becomes warm.
- When the initial reaction subsides, add another 1.5 ml of HCl.
- Continue the reaction with periodic additions of HCl.
- If the reaction becomes too vigorous, briefly cool the flask in cold water.
- Keep the mixture well shaken.
- Heat the mixture on a boiling water bath for 30-60 minutes, until the odor of nitrobenzene disappears.
- A white or yellow crystalline complex, aniline chlorostannate, may form during cooling.
- Cool the reaction mixture to room temperature
- Extract it with three volumes of 2.5 ml of ethyl acetate.
- Collect the extract and evaporate it to dryness.
- Recrystallize the residue using ethanol.
Calculation
Now calculate the values by these equations

This is about the laboratory preparation and synthesis of benzocaine, which is used as local anesthetic.
You may also like to read How to prepare 2,3-diphenyl quinoxaline from benzyl?
