Learn the principles of UV-Visible spectroscopy and its applications. UV spectroscopy is used in chemistry, biology and materials science.
What is UV-visible spectroscopy?
It is a powerful analytical technique which helps scientists to study the electronic transitions of molecules.
We can measure the absorption of light in the ultraviolet (UV) and visible (Vis) regions of the electromagnetic spectrum. UV spectroscopies give insight of electronic structure and properties of a wide range of samples.
Principle of UV visible spectroscopy
This technique of spectroscopy follows the Beer-Lambert Law, which describes about the relationship between the concentration, the path length of the light through the sample and the absorbance of the sample.
A UV-Visible spectrophotometer measures the amount of light that is absorbed by a sample over a range of wavelengths.
We can use this data to get knowledge about the electronic structure and properties of the sample.
Beer-Lambert law
The Beer-Lambert-Bouguer law is a fundamental law of spectrophotometry. It relates the concentration of a sample to its absorbance or transmission. The equation for the Beer-Lambert law is as follows: A = εcl
- A is the absorbance of the sample.
- ε is the molar absorptivity or extinction coefficient of the substance at a given wavelength.
- c is the concentration of the substance in solution (in moles per liter).
- l is the path length of the sample (in centimeters).
The Beer-Lambert law states that the absorbance of a sample is directly proportional to its concentration and path length. This law is widely used in UV-Visible spectroscopy and other forms of spectrophotometry.
It allows scientists to accurately determine the concentration of a sample from its absorbance or transmission spectrum.
Instrumentation
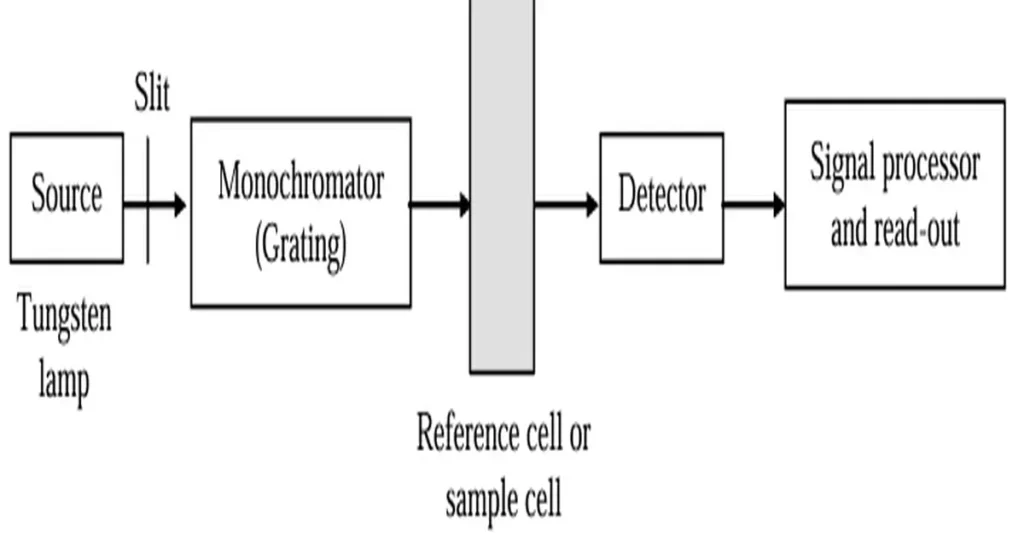
The essential parts of a uv spectrophotometer are:
- Source of radiation.
- Wavelength selector.
- Cells or cuvettes.
- Detector and photomultiplier tubes.
- Recording system.
- Power supply.
Source of radiation
A UV-visible spectrophotometer starts with a light source. It may consist of typically a tungsten filament for visible light and a deuterium or xenon lamp for UV light.
Wavelength selectors
It includes absorption filters, interference filters, monochromators and slits. This setup ensures accurate control of wavelengths. These are crucial for studying absorption patterns in materials.
Cells or cuvettes
These are sample containers. These cells or cuvettes contain quartz for UV (below 350nm) and silicate glass for visible light.
Detectors and photomultiplier tubes
The most commonly used detectors in uv spectrophotometer are:
- Phototubes or photo-emissive cells.
- Photomultiplier tubes (PMT).
- Photovoltaic cell or barrier-layer cells.
- Photomultiplier silicon photodiode array detector.
Recording system
In UV spectrophotometers, the signal from the photomultiplier tube is received by a recording system. This is typically containing a recorder pen for data representation.
Power supply
The power supply serves three main functions:
- Voltage reduction with a transformer.
- AC to DC conversion with a rectifier.
- Ripple smoothing for constant voltage supply.
UV-visible spectroscopy application
- UV-Visible spectroscopy used to study electronic properties of chemical reaction.
- Used to measure concentration and purity of sample.
- Characterizes optical properties of dyes, pigments and nanoparticles.
- We can monitor quality of chemicals by measuring concentrations.
- Used in pharmaceuticals for development and ensuring purity.
- Used to monitoring drug stability.
Also read Conductometry definition principle instrumentation application.
FAQ’s
What is the advantage of uv-visible spectroscopy?
UV-Visible spectroscopy is a quick and sensitive spectroscopic technique.
What is uv-visible spectroscopy principle?
UV-visible spectroscopy measures the absorption of ultraviolet and visible light by molecules. This measurement done by the electronic transitions within molecules. By studying these absorbed colors, we can learn about the substance and its concentration.
