Learn what is quality management system and certification with definition and what are the elements of quality management systems.
QMS is an important aspect for the pharmaceutical industry for maintaining the quality and safety for their products and services.
Quality Management System
Quality Management System (QMS) is a set of policies, processes, documented procedures and records that define how an organization ensures the quality of its products or services.
Also, it can be defined as, QMS is a set of policies, processes and procedures required for planning and execution (production, development or service) in the core business area of an organization. For example, ISO 9001.
What is certification in QMS?
In quality management system certifications are like badges of excellence that help organizations get better. Getting a QMS certification means that a business has a documented system for making high-quality products.
What are the benefits of QMS certification?
- Ensures quality for customer satisfaction.
- Attracts new customers and increase growth.
- Applying for government tenders.
- Establishes efficient documentation and procedures.
- Enhances internal and external communication.
- Identifies efficiencies, reducing costs and waste.
- Promotes a positive and efficient work culture.
What is ISO 9001:2015?
ISO 9001:2015 is the primary quality management certification. It sets out the standard criteria for a QMS that can be used by a large or small organization, irrespective of its field of activity. This standard is based on a number of quality management principles, i.e., a strong customer focus, motivation and implication of top management, process approach, thinking based on risks and opportunities and achieving continual improvement. By implementing the ISO 9001:2015 standard an organization can provide quality products or services and build customer confidence is demonstrated.
Concept of quality
According to US FDA, Quality can be defined as, a measure of a products or services ability to satisfy the customers stated or implied needs.
The word quality has different meanings. Quality includes “fitness for use,” “customer satisfaction,” “doing things right the first time,” or “zero defects.”
These definitions are accepted as quality refers to degrees of excellence. Quality is defined as “an inherent characteristic, property or attribute”. Quality control is the science that keeps these characteristics or qualities within the limit range.
In a manufacturing, quality of design and quality of conformance are the two major categories of quality.
What are Quality Systems?
A quality system is like a guide for making sure things are good. It helps to follow the right steps to keep products standards. The rules are written down in a quality manual, with all the details in other documents. Quality systems involve the three basic elements quality management, quality control and quality assurance.
What is quality management?
Quality Management is how we put our best quality plans into action. It involves planning and overseeing activities to control and assure quality. The goal of QM is to make sure everything meets the quality standards. It maintains effectiveness by reviewing the quality system and also identifies any insufficiencies.
What is quality control?
QC is like the inspector for a company. It uses different methods to check if products or services meet the standards. It also helps to determine how things are done and also it recommends the ways to make them better.
What is quality assurance in pharmacy?
QA is like the bodyguard for a product or service. It involves planned and systematic activities to make assuring that a product or service will meet the specifications.
The major difference between QA and QC is quality control focuses on making a good product and quality assurance ensures it stays that way.
What are the elements of quality management systems?
These are the elements of QMS, Total Quality Management (TAM), Quality by design, Six Sigma concept, Out of Specifications (OOS), Change control, Introduction to ISO 9000 series of quality systems standards, ISO 14000, NABL and GLP.
What is total quality management (TQM)?
Total Quality Management (TQM) is a management approach which focused on enhancing the quality and productivity of a business. It is a strategy that collaborates across all organization departments and involves employees, suppliers and clients/customers to aiming for product improvement.
TQM involves the following eight principles:
- Leadership.
- Customer focus.
- Process approach.
- Involvement of people.
- System approach.
- Continuous improvement.
- Factual approach to decision Making.
- Mutually beneficial supplier relationships.
What is quality by design?
Quality by Design (QbD) is a concept introduced by Dr. Joseph M. Juran. It emphasizes that quality should be integral to product design to prevent issues.
QbD involves designing and developing formulations and manufacturing processes that ensure predefined product specifications. This concept has been lately adopted in the pharmaceutical industries through several initiatives e.g., ICH Q81, Q92 and Q103, new regulatory documents, Process Analytical Technology (PAT)5, FDAs cGMP for the 21st Century.
QbD focuses on understanding of processes and products, leading to improvements in product quality, process efficiency and regulatory flexibility.
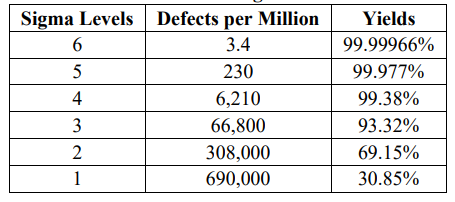
What is six sigma concept?
In statistics, the term Sigma represents standard deviation that indicates the degree of variation in a set of measurements or a process.
Six sigma is a statistical concept, or a quality management method used for the measurement of a process or a product in terms of defects at the six-sigma level. The concept also focuses on developing and delivering perfect products and services.
Carl Frederick Gauss (1777-1855) who gave the concept of normal curve. The Six Sigma concept came in to force as a measurement standard.
This concept is being used in product variation as a measurement standard since the 1920‟s, when Walter Shewhart described that three sigma from the mean is the point where a process requires correction.
Later, many measurement standards (Cpk, Zero Defects, etc.) came into existence. The term Six Sigma was described by Bill Smith (a Motorola engineer). Six Sigma is a federally registered trademark of Motorola.
What is out of specifications (OOS)?
Out of Specifications (OOS) refers to test results during the making or testing of a product that don’t meet the specified limits in official documents like compendia, drug master files or drug applications.
OOS can happen due to issues in manufacturing, errors in testing or problems with analytical equipment. To understand why it occurred, a root cause analysis is conducted to investigate the reasons for the OOS.
What is change control?
Change is an essential part of a pharmaceutical product life cycle. A change involves an addition to, deletion of, or modification to manufacturing facility, utilities, process, material, product, procedures or equipment (including software) that influences quality or regulatory requirements.
Change control in CGMP (Current Good Manufacturing Practice) is a concept that handles changes to prevent unintended consequences. Certain manufacturing changes, like alterations to specifications, critical product attributes or bioavailability may be made after regulatory filings but before regulatory approval.
Change control aims to prevent the unintended consequences that may be encountered when changing a product or system.
What is ISO 9000 in quality management?
ISO 9000 is a set of international standards focusing on quality management and assurance. It helps companies in systematically documenting quality system elements for effective implementation and maintenance of an efficient quality system.
These standards are versatile, not tied to any specific industry. These standards can be applied to organizations of any size, be it big or small. Some examples of ISO 9000 standards are, ISO 8402, ISO 9000-1, ISO 9000-2 and ISO 9000-3 etc.
What is ISO 14000 in quality management system?
The ISO 14000 is a family of standards which offers practical tools for companies and organizations to handle their environmental responsibilities. For example, ISO 14001:2015 and ISO 14006:2011 concentrate on environmental systems.
Other standards in the ISO 14000 series address areas like audits, communications, labeling, life cycle analysis and environmental challenges such as climate change. These standards are developed by the ISO Technical Committee (ISO/TC) 207 and its sub-committees.
What is GLP?
GLP stands for Good Laboratory Practice. It is a set of quality systems and standards that are followed by laboratories to ensure the consistency, reliability and integrity of test data. GLP is particularly important in industries such as pharmaceuticals, chemicals and biotechnology.
What is NABL?
National Accreditation Board for Testing and Calibration Laboratories is a constituent board of the Quality Council of India. It offers accreditation to conformity assessment bodies through third-party assessments. This accreditation specifically focuses on assessing the technical competence of various entities, including testing laboratories (including medical labs), calibration laboratories, proficiency testing providers and producers of reference materials.
NABL aims to provide a recognized and credible framework for assessing and ensuring the quality and competence of these bodies.
This is all about Quality Management System in industrial pharmacy.
Also read Regulatory Requirements for Drug Approval. Drug regulatory authorities in pharmacy with list.

